Graphene batteries: Introduction and Market News
Graphene and batteries
Graphene, a sheet of carbon atoms bound together in a honeycomb lattice pattern, is hugely recognized as a wonder material due to the myriad of astonishing attributes it holds. It is a potent conductor of electrical and thermal energy, extremely lightweight chemically inert, and flexible with a large surface area. It is also considered eco-friendly and sustainable, with unlimited possibilities for numerous applications.
The advantages of graphene batteries
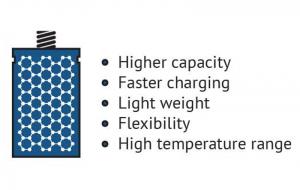
In the field of batteries, conventional battery electrode materials (and prospective ones) are significantly improved when enhanced with graphene. A graphene battery can be light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery's life, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries.
Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries (and other types of rechargeable batteries) can be enhanced by introducing graphene to the battery's anode and capitalizing on the material's conductivity and large surface area traits to achieve morphological optimization and performance.
It has also been discovered that creating hybrid materials can also be useful for achieving battery enhancement. A hybrid of Vanadium Oxide (VO2) and graphene, for example, can be used on Li-ion cathodes and grant quick charge and discharge as well as large charge cycle durability. In this case, VO2 offers high energy capacity but poor electrical conductivity, which can be solved by using graphene as a sort of a structural backbone on which to attach VO2 - creating a hybrid material that has both heightened capacity and excellent conductivity.
Another example is LFP (Lithium Iron Phosphate) batteries, that is a kind of rechargeable Li-ion battery. It has a lower energy density than other Li-ion batteries but a higher power density (an indicator of of the rate at which energy can be supplied by the battery). Enhancing LFP cathodes with graphene allowed the batteries to be lightweight, charge much faster than Li-ion batteries and have a greater capacity than conventional LFP batteries.
In addition to revolutionizing the battery market, combined use of graphene batteries and graphene supercapacitors could yield amazing results, like the noted concept of improving the electric car's driving range and efficiency. While graphene batteries have not yet reached widespread commercialization, battery breakthroughs are being reported around the world.
Battery basics
Batteries serve as a mobile source of power, allowing electricity-operated devices to work without being directly plugged into an outlet. While many types of batteries exist, the basic concept by which they function remains similar: one or more electrochemical cells convert stored chemical energy into electrical energy. A battery is usually made of a metal or plastic casing, containing a positive terminal (an anode), a negative terminal (a cathode) and electrolytes that allow ions to move between them. A separator (a permeable polymeric membrane) creates a barrier between the anode and cathode to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current. Finally, a collector is used to conduct the charge outside the battery, through the connected device.
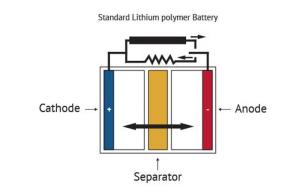
When the circuit between the two terminals is completed, the battery produces electricity through a series of reactions. The anode experiences an oxidation reaction in which two or more ions from the electrolyte combine with the anode to produce a compound, releasing electrons. At the same time, the cathode goes through a reduction reaction in which the cathode substance, ions and free electrons combine into compounds. Simply put, the anode reaction produces electrons while the reaction in the cathode absorbs them and from that process electricity is produced. The battery will continue to produce electricity until electrodes run out of necessary substance for creation of reactions.
Battery types and characteristics
Batteries are divided into two main types: primary and secondary. Primary batteries (disposable), are used once and rendered useless as the electrode materials in them irreversibly change during charging. Common examples are the zinc-carbon battery as well as the alkaline battery used in toys, flashlights and a multitude of portable devices. Secondary batteries (rechargeable), can be discharged and recharged multiple times as the original composition of the electrodes is able to regain functionality. Examples include lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics.
Batteries come in various shapes and sizes for countless different purposes. Different kinds of batteries display varied advantages and disadvantages. Nickel-Cadmium (NiCd) batteries are relatively low in energy density and are used where long life, high discharge rate and economical price are key. They can be found in video cameras and power tools, among other uses. NiCd batteries contain toxic metals and are environmentally unfriendly. Nickel-Metal hydride batteries have a higher energy density than NiCd ones, but also a shorter cycle-life. Applications include mobile phones and laptops. Lead-Acid batteries are heavy and play an important role in large power applications, where weight is not of the essence but economic price is. They are prevalent in uses like hospital equipment and emergency lighting.
Lithium-Ion (Li-ion) batteries are used where high-energy and minimal weight are important, but the technology is fragile and a protection circuit is required to assure safety. Applications include cell phones and various kinds of computers. Lithium Ion Polymer (Li-ion polymer) batteries are mostly found in mobile phones. They are lightweight and enjoy a slimmer form than that of Li-ion batteries. They are also usually safer and have longer lives. However, they seem to be less prevalent since Li-ion batteries are cheaper to manufacture and have higher energy density.
Batteries and supercapacitors
While there are certain types of batteries that are able to store a large amount of energy, they are very large, heavy and release energy slowly. Capacitors, on the other hand, are able to charge and discharge quickly but hold much less energy than a battery. The use of graphene in this area, though, presents exciting new possibilities for energy storage, with high charge and discharge rates and even economical affordability. Graphene-improved performance thereby blurs the conventional line of distinction between supercapacitors and batteries.
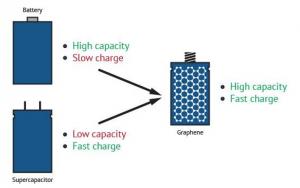 Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene-enhanced batteries are almost here
Graphene-based batteries have exciting potential and while they are not yet fully commercially available yet, R&D is intensive and will hopefully yield results in the future. Companies all over the world (including Samsung, Huawei, and others) are developing different types of graphene-enhanced batteries, some of which are now entering the market. The main applications are in electric vehicles and mobile devices.
Some batteries use graphene in peripheral ways - not in the battery chemistry. For example in 2016, Huawei unveiled a new graphene-enhanced Li-Ion battery that uses graphene to remain functional at higher temperature (60° degrees as opposed to the existing 50° limit) and offer a double the operation time. Graphene is used in this battery for better heat dissipation - it reduces battery's operating temperature by 5 degrees.
Further reading
- Introduction to graphene
- Graphene Supercapacitors
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
- Graphene-Info's graphene batteries market report
- Graphene supercapacitors market report
Komaki launches electric scooter that is said to use graphene battery
It was reported that Indian electric vehicle brand Komaki has introduced the new model of Cat 3.0 NXT that comes with two battery variants, Graphene and LIPO4, and will be available for Rs. 1,19,999 (around USD$1400) and Rs. 1,49,999 (almost USD$1800). The unveiling of this EV is aimed at last-mile delivery operators, enabling sustainable all-day use and supporting SMEs and MSMEs in growing their businesses.
The EV features app-based battery options, Graphene and LIPO4, giving a range of over 180 km to 200 km on a single charge, depending on the battery type.
Graphene Manufacturing Group engages with Australian government on battery solutions
Graphene Manufacturing Group (GMG) has provided an update on its ongoing engagement with the Australian Federal Government, highlighting recent meetings with key officials.
GMG’s leadership, including CEO Craig Nicol, met with Senator Tim Ayres, Assistant Minister for Trade, to discuss the Company’s battery manufacturing progress and how federal policies like the Future Made in Australia Battery Breakthrough could benefit GMG. Further discussions took place with Speaker of the House of Representatives Milton Dick and Queensland Senator Anthony Chisholm, building on prior visits to GMG's facilities in Richlands, Queensland.
Lyten plans $1B Lithium-Sulfur battery Gigafactory in Nevada
U.S-based Lyten announced plans to invest more than $1 billion to build the world’s first Lithium-Sulfur battery gigafactory. The facility will be located near Reno, Nevada, and will have the capability to produce up to 10 GWh of batteries annually at full scale. Phase 1 of the facility is scheduled to come online in 2027.
Lyten’s proprietary processes permanently sequester carbon from methane in the form of 3D Graphene and utilize the supermaterial to develop decarbonizing applications. Lyten has received more than $425 million in investment from companies including Stellantis, FedEx, Honeywell, Walbridge, the European Investment Fund, and the Luxembourg Future Fund.
Infinity Turbine introduces 3D-printed electrodes for Salgenx saltwater batteries and electrocatalyst applications
Infinity Turbine, developer of sustainable energy storage solutions, has unveiled an approach to electrode fabrication that combines fiber laser heat treating, 3D additive manufacturing, and laser-induced carbonization. This synergistic technology enables the direct transformation of carbon-rich materials like sugar and wood fibers (including bamboo) into hard carbon or graphene-like structures. The resulting 3D-printed electrodes are set to revolutionize the manufacturing of Salgenx saltwater flow batteries, gas processing, and electrocatalyst applications.
Salgenx is developing saltwater flow batteries as a solution for safe, environmentally friendly grid-scale energy storage. With the introduction of 3D-printed carbon electrodes, Infinity Turbine can enhance the battery’s efficiency by providing a high-conductivity, high-surface-area electrode structure. The combination of laser-induced graphene and tailored 3D-printed geometries reportedly allows for faster ion exchange, improved energy density, and longer battery life, all while using sustainable, carbon-rich materials. The concept of a 3D printed electrode reduces manufacturing time and complexity, resulting in more efficient electrode production with just-in-time (JIT) technology integration and decreased inventory costs.
Manchester University team discovers energy storage mechanism in bi-layer graphene anode
A team of scientists from the University of Manchester has gained new understanding of lithium-ion storage within the thinnest possible battery anode - composed of just two layers of carbon atoms. Their work shows an unexpected ‘in-plane staging’ process during lithium intercalation in bilayer graphene, which could pave the way for advancements in energy storage technologies.
Lithium-ion batteries, which power everything from smartphones and laptops to electric vehicles, store energy through a process known as ion intercalation. This involves lithium ions slipping between layers of graphite - a material traditionally used in battery anodes, when a battery is charged. The more lithium ions that can be inserted and later extracted, the more energy the battery can store and release. While this process is well-known, the microscopic details have remained unclear. The Manchester team’s discovery sheds new light on these processes by focusing on bilayer graphene, the smallest possible battery anode material.
Graphene Manufacturing Group gives update, says its Richlands plant "exceeds hopes"
Graphene Manufacturing Group (GMG) has provided a business update on its recently commissioned modular graphene production plant at Richlands, Australia. The graphene production plant has been operating and producing graphene since its commissioning date in December, 2023.

The performance of the production unit, according to GMG's CEO and managing director, Craig Nicol, has "exceeded the Company's expectations in both graphene production rate and graphene quality. The company continues to perform minor optimizations with this new production plant which have both increased production yield and quality of the graphene".
Researchers develop scalable graphene technology for regulating heat transfer and enhancing battery safety
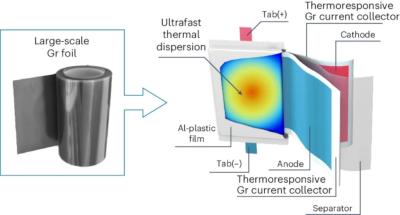
Researchers at Swansea University, in collaboration with China's Wuhan University of Technology and Shenzhen University, have developed a technique for producing large-scale graphene current collectors that could significantly enhance the safety and performance of lithium-ion batteries (LIBs).
Their recent study details the first successful protocol for fabricating defect-free graphene foils on a commercial scale. These foils offer excellent thermal conductivity - nearly ten times higher than traditional copper and aluminium current collectors used in LIBs.
Danish Graphene completes setup of its battery laboratory
Danish Graphene has announced that its battery laboratory is now fully setup and operational. The Company sees this as a significant milestone, as it now has the capability to produce and test batteries containing graphene, entirely in-house.
With this new battery laboratory, Danish Graphene can manage the whole process internally – from production of individual components to the final assembly of coin cell batteries. This allows the Company to maintain strict quality control and explore new technologies and materials.
Cerebral Energy and Cornell University secure funding for lithium-free aluminum-graphene batteries to support Air Force Special Operations
Cerebral Energy has announced it has been selected by AFWERX (the innovation arm of the U.S Air Force, powered by the Air Force Research Laboratory (AFRL)) for a Phase II STTR follow-on contract in the amount of $1.6 million to support further development of a new lithium-free secondary battery using recycled aluminum and graphene derived from recycled US waste streams.
The technology was developed by Dr. Lynden Archer - Dean of the School of Engineering at Cornell University and licensed by Cerebral. The novel aluminum battery design is over 3X more efficient than lithium, and said to be much safer (no fire risk), 10X faster charging and has no supply chain challenges since the materials are derived from US waste streams. Known as "AGILE," the batteries will first support Air Force Special Operations Command (AFSOC) medical modernization teams to address their pressing tactical power issues.
First Graphene secures funding for a research project with Swansea University to determine the market potential of the Company’s Kainos Technology
First Graphene has secured funding for a collaborative research project with Swansea University to determine the market potential of the Company’s Kainos Technology.

The grant is valued at approximately AU$192,152 (over USD$130,000) and was secured through Analysis for Innovators (A4i) Round 12, Stage 2 funding, delivered by Innovate UK. The funding is specifically meant for businesses utilizing expertise from leading research facilities across the UK to overcome
productivity or technical barriers of new technologies towards market readiness.
Pagination
- Page 1
- Next page