Researchers from Göttingen and Pasadena (USA) have produced an "atomic scale movie" showing how hydrogen atoms chemically bind to graphene in one of the fastest reactions ever studied. The team found that by adhering hydrogen atoms to graphene, a bandgap can be formed.
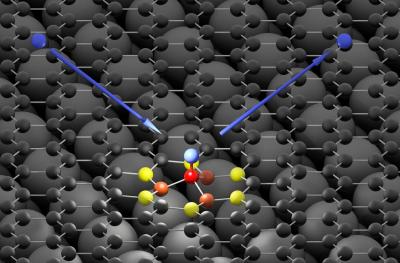 The hydrogen atom (blue) hits the graphene surface (black) and forms a bond with a carbon atom (red). The high energy of the hydrogen atom is first absorbed by neighboring carbon atoms (orange and yellow) and then passed on to the graphene as a sound wave
The hydrogen atom (blue) hits the graphene surface (black) and forms a bond with a carbon atom (red). The high energy of the hydrogen atom is first absorbed by neighboring carbon atoms (orange and yellow) and then passed on to the graphene as a sound wave
The research team bombarded graphene with hydrogen atoms. "The hydrogen atom behaved quite differently than we expected," says Alec Wodtke, head of the Department of Dynamics at Surfaces at the Max Planck Institute (MPI) for Biophysical Chemistry and professor at the Institute of Physical Chemistry at the University of Göttingen. "Instead of immediately flying away, the hydrogen atoms 'stick' briefly to the carbon atoms and then bounce off the surface. They form a transient chemical bond," Wodtke exclaims. Something else also surprised the scientists: The hydrogen atoms have a lot of energy before they hit the graphene, but not much left when they fly away. It seems that hydrogen atoms lose most of their energy on collision, but where it goes remained to be examined.





