Researchers from KTH Royal Institute of Technology, University of New South Wales and University of Milan have a reported a reproducible and scalable method for producing graphene oxide (GO) nanosheets from commercial carbon fibers. The process involves exfoliating carbon fibers with nitric acid, which reportedly provides high yields of one-atom-thick sheets of graphene oxide with characteristics comparable to commercial GO sourced from mined graphite.
The GO production process, from commercial carbon fibers to graphene sheets. Image from: Small
The team explained that the proof of concept was carried out with carbon fibers derived from polyacrylonitrile (PAN), a widely available polymer that undergoes high-temperature oxidation and graphitization. The method could be duplicated with other raw sources, such as raw sources such as biomass or forest industry sidestreams.
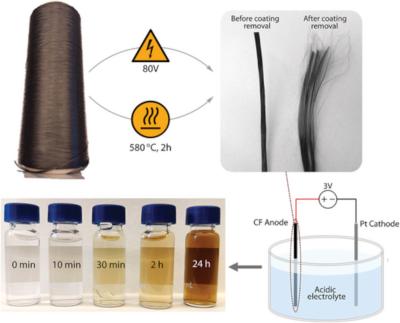
The method consists of transforming the carbon fibers using the process of electrochemical oxidation in a bath of water and nitric acid. The bath acts as a conductor and when an electric current is sent through carbon fiber, the material begins to lose electrons which transforms the surface much the same way that oxidization appears as rust on a car. In this case, the transformation causes layers of nanoscale graphene oxide to peel off from the carbon fibers' surface.
The study discovered a window in which just 5 percent nitric acid was perfect for creating these tiny nanosheets, ranging from 0.1 to 1 micrometer in size, with a uniform thickness of about 0.9 nanometers. Notably, the GO nanosheets synthesized this way emerged in circular and elliptical shapes, in contrast to the polygonal shapes typical of GO synthesized from natural, mined graphite.
Synthesis of GO from mined graphite requires harsh chemicals and often results in material inconsistencies due to variations in graphite purity. Compared to existing synthetic methods, the new approach delivers a high yield of 200 milligrams of GO per gram of carbon fiber. This efficient conversion rate makes it viable for large-scale production, addressing a key challenge in nanomaterial synthesis.
To ensure the nanosheets met quality standards, the researchers examined and measured the properties and structure of the material with a number of advanced techniques.
The study also explored methods to remove protective polymer coatings from commercial carbon fibers before oxidation, heating at 580 °C for two hours and shock-heating to 1200 °C for three seconds—both proving effective. The research demonstrated that the nature of electrical conduction within the fibers plays a crucial role in the electrochemical exfoliation process.
The next steps for the researchers include investigating biobased sources for carbon fibers, delving deeper into how the process works.