Researchers at the California Institute of Technology (Caltech) have developed a method for coating lithium-ion battery cathodes with graphene, extending their life and performance. This recent effort may improve lithium-ion battery performance and reduce reliance on cobalt, an element frequently used in lithium-ion batteries that is difficult to source sustainably.
Image credit: Caltech
Caltech senior research scientist, David Boyd, has worked over the past decade to develop techniques for manufacturing graphene. In 2015, Boyd and colleagues discovered that high-quality graphene could be produced at room temperature. Prior to this, the production of graphene required extremely high temperatures, up to 1,000 degrees Celsius. After this breakthrough, they searched for new applications for graphene. Recently, Boyd teamed up with Will West, a technologist at Caltech's Jet Propulsion Laboratory (JPL), which Caltech manages for NASA. West specializes in electrochemistry and, in particular, in the development of improved battery technologies. Boyd and West set out to see if graphene could create an improved lithium-ion battery, which they have shown to be possible.
"Demonstrating a reliable trend in battery-cell performance requires consistent materials, consistent cell assembly, and careful testing under a range of conditions," says Brent Fultz, Caltech's Barbara and Stanley R. Rawn, Jr., Professor of Materials Science and Applied Physics. "It is fortunate that the team was able to do this work so reproducibly, although it took some time to be sure."
Despite its ubiquity, there is room for improvement in lithium-ion battery technology. For example, Boyd says, "Tesla engineers want a cost-effective battery that can charge quickly and operate for a longer period of time between charges. That's called the charge-rate capability."
West adds: "The more times you can charge a battery over its lifetime, the fewer batteries you have to use. This is important because lithium-ion batteries make use of limited resources and disposing of lithium-ion cells safely and effectively is a very challenging task."
An important feature of lithium-ion batteries is how they perform after many cycles of charging and use. Batteries work by creating chemical energy between the two ends of the battery—the cathode and the anode—and converting it into electrical energy. As the chemicals in the cathode and those in the anode function over time, they may not fully recover to their original condition. A common problem is the dissolution of transition metals from the cathode material which is particularly severe in cathode materials with high manganese content, though less so for high cobalt content cathode materials.
"As a result of unwanted side-reactions that occur during cycling, transition metals in the cathode gradually end up in the anode where they get stuck and reduce the performance of the anode," Boyd explains. This transition metal dissolution (TMD) is a reason why expensive cobalt-bearing cathodes are used instead of inexpensive high manganese content cathodes.
A further challenge for lithium-ion batteries is that they require metals that are expensive, scarce, and not always mined responsibly. A significant amount of the global supply of cobalt, in particular, is concentrated in the Democratic Republic of the Congo, and much of that cobalt is extracted by so-called artisanal miners: freelance workers, including children, who engage in dangerous and demanding physical labor for little to no pay.
The search has been on for ways to increase battery performance while also reducing or eliminating the use of cobalt and still preventing TMD.
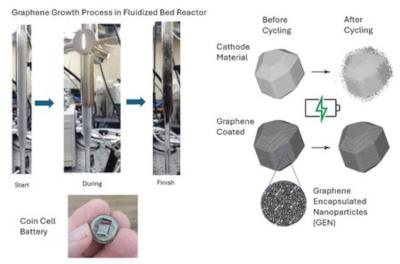
Engineers previously knew that carbon coatings on a lithium-ion battery's cathode could slow or stop TMD, but developing a method to apply these coatings proved difficult. "Researchers have tried to deposit graphene directly onto the cathode material, but the process conditions typically needed to deposit graphene would destroy the cathode material," Boyd explains. "We investigated a new technique for depositing graphene on the cathode particles called dry coating. The idea is that you have one 'host' substance of large particles and a 'guest' substance of tiny particles. By mixing them under certain conditions, the system can undergo a phenomenon known as 'ordered mixing' in which the guest particles uniformly coat the host particles."
Dry-coating technology has been in use since the 1970s in the pharmaceutical industry to extend the life of tablets by protecting them from moisture, light, and air.
Boyd recalls thinking "This is a good idea we might be able to use with graphene! We can first manufacture graphene guest particles—graphene encapsulated nanoparticles (GEN)—using our room-temperature method, and then dry coat a very small amount of it (1 percent in weight) onto the host cathode material so that graphene effectively covers and protects the cathode."
Dry coating the cathode with a graphene composite proved successful in the lab. The graphene coating sharply reduced TMD, simultaneously doubled battery cycle life, and allowed the batteries to function across a somewhat wider temperature range than previously possible. This result surprised researchers. It was assumed that only a continuous coating could suppress TMD and that a dry coating composed of particles could not. In addition, because graphene is a form of carbon, it is widely available and environmentally friendly.
This method has additional benefits for the battery industry. "Battery factories are very expensive. A lot of money has been invested into them," Boyd says. "So it's very important that improved battery technologies are scalable and can fit into the workflows of existing battery manufacturing. We can take almost any cathode material and add in just a small amount of our GEN, run it for a few minutes in the dry mixer, and it will reduce transition metal dissolution and improve charge-rate capacity."
"This is also an advance for coating technologies in general," Boyd says. "It opens up a lot of possibilities for the use of dry coatings."