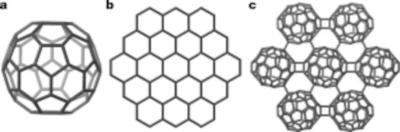
A team of researchers from Columbia University, University of Virginia, University of Rhode Island, Amherst College, Barnard College and Harvard University have discovered a new type of carbon material: graphullerene.
The material is a new 2D form of carbon made up of layers of linked fullerenes peeled into ultrathin thin flakes from a larger graphullerite crystal—similarly to the way graphene is peeled from crystals of graphite.
"It is amazing to find a new form of carbon in the year 2022," said Columbia University's Colin Nuckolls. "It also makes you realize that there is a whole family of materials that can be made in a similar way that will have new and unusual properties as a consequence of the information written into the superatomic building blocks."
Elena Meirzadeh from Columbia University, who synthesized the first crystals of graphullerite, referred to graphullerene as the superatomic "cousin" of graphene. Unlike graphene and most other two-dimensional materials that are made of repeating elements that are limited to specific bonding geometries and have specific properties as a result, graphullerene's superatomic structure makes it incredibly modular, she explained. With 60 carbon atoms in the ball to work with, fullerenes can theoretically be linked together in a number of different ways, each of which could yield different electronic, magnetic, and optical properties—this first version represents just one possible configuration, according to the team.
It's a new way of thinking about structures and their properties as they grow, added Columbia University's Michael L. Steigerwald. "For more than 30 years, researchers have had the notion that clusters of atoms will behave differently than the larger solids that they form," he said. "Here, we are making a solid out of an existing carbon superatom to see how that kind of organization will influence its properties. Would the new material behave like fullerene, or like something else?"
The team set out to bond fullerenes molecules rather than individual carbon atoms into a layered, peelable crystal, in order to study its superatomic properties in two dimensions. Meirzadeh used a high-temperature solid-state synthesis technique involving a magnesium scaffold that was later removed—a process involving acid that, after a year spent working with air-sensitive crystals inside a glovebox, was a somewhat stressful final step. "As chemists, we try things and don't always know what will happen. I thought it would fall apart, but it remained intact," she recalled. "Seeing an intact, pure carbon crystal that we could then easily exfoliate and study was a great surprise."
Once the new material was made, Meirzadeh sent samples off to collaborators at Columbia and beyond for initial imaging and characterization. The battery of tests reportedly revealed a number of intriguing electrical, optical, and thermal properties. Like graphene, graphullerene can confine and polarize light, it can accept lots of extra electrons, and it can form tunable superlattice structures; these properties make it a promising material with potential applications in new kinds of optical and electronic devices. Compared to fullerenes, graphullerite crystals are shown to have a much higher thermal conductivity, a result of the strong covalent bonds within each graphullerene sheet. Thermal conductivity helps dissipate heat, an important consideration when building devices.
The work is a starting point for exploring the potential of graphullerene. From a chemistry perspective, the researchers plan to tweak and tune its modular properties and introduce new structures, while collaborators will look more closely at what happens when graphullerene sheets are combined with different kinds of two-dimensional materials studied at Columbia to better understand the possibilities.