Researchers from Tecnologico Nacional de México, McGill University and Centro de Investigacion en Materiales Avanzados S.C. have examined the use of Graphene nanobuds (GNBs), formed on copper (Cu) foil via chemical vapor deposition, as an anode in lithium-ion batteries.
Producing high-performance anode materials that possess excellent specific capacities and extended cyclic ability is currently one of the key development areas in lithium-ion batteries. The energy storage capacity of lithium-ion batteries heavily depends on the anode materials used and their structure. Carbonaceous substances remain the anode materials of choice because of the strong attraction between lithium (Li) and graphitic carbon. Graphene possesses a large capacity to accommodate lithium ions in its framework because of its significant surface area and excellent electrical conductivity.
Graphene is often synthesized by chemically exfoliating graphite or reducing graphite oxide; such methods are very cost-effective and practical.
Reduced graphene oxide (rGO) has relatively high capacity levels. When used as an anode, rGO exhibits a large irreversible capacity. This ultimately means that the accumulated Li never entirely recovers following Li injection, leading to poor coulombic efficiency in the initial cycle. Therefore, using graphene oxide in lithium-ion batteries has proved to be challenging thus far.
Additional carbon-based nanomaterials may be introduced to graphene to boost its energy storage capacity and cyclic stability and improve its anode suitability in lithium-ion batteries. Energy storage capacity may be enhanced by the covalent bonding of fullerenes on the surface of graphene. Incorporating fullerenes with graphene is an effective technique for developing a novel nanostructure with strong electrocatalytic capabilities.
Unique three-dimensional structures called graphene nanobuds are formed by coupling these carbon-based materials. While this composite material has demonstrated remarkable electrolytic characteristics, its full potential in lithium-ion battery applications is yet to be explored.
This recent study describes the production of a graphene nanobud composite, which exhibited remarkable electrolytic energy storage capabilities.
A customized CVD process was used to fabricate the graphene nanobuds in a singular step. The team used Cu foil for the current collection at the anode of a lithium-ion battery. The study focused on fabricating graphene nanobuds deposited on a copper platform.
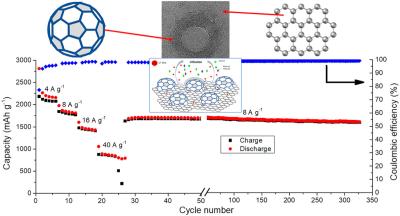
A customized chemical vapor deposition technique was used to create few-layer and multilayer GNBs. These graphene nanobuds showed excellent electrolytic characteristics, highlighting their potential as anode materials in lithium-ion batteries.
The few-layer GNBs outperformed the multilayer GNBs in terms of electrochemical performance, exhibiting a better energy storage capacity, a higher current rate, and outstanding cycle stability, all while maintaining a faradaic efficiency of 99 %.
The developed graphene nanobuds had the inherent qualities of improving lithium-ion storage and diffusion. These inherent qualities are responsible for the improved capacity, better reversibility, and outstanding cycle performance exhibited by the graphene nanobuds.
Directly growing the active material on the copper current collector enabled the electrode to retain excellent electrical conduction and superior rate capability, leading to highly improved electrolytic qualities for high-performance lithium-ion batteries.
These findings can pave the way for future work on the design of high-performance carbonaceous anode materials for cutting-edge lithium-ion batteries.



