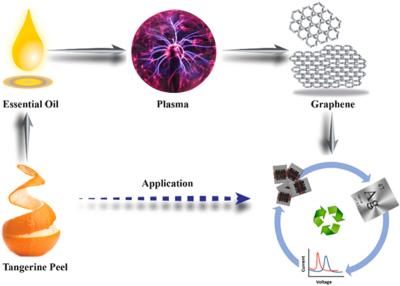
In 2015, scientists at James Cook University in Queensland, Australia, and collaborators from institutions in Australia, Singapore, Japan, and the US developed a technique for growing graphene from tea tree extract. Now, scientists from James Cook University developed a process to synthesize graphene from tangerine peel oil, which they then used to recover silver from waste PV material. To demonstrate the quality of the recovered silver and the synthesized graphene, they made a dopamine sensor that reportedly outperformed reference devices.
The team synthesized “freestanding” graphene using non-toxic and renewable tangerine peel oil that can reportedly be used for the recovery of silver from end-of-life organic PV devices. The researchers said that their process result in high-quality graphene and demonstrated a remarkable ability to selectively recover silver from photovoltaic waste. One of the most surprising findings, according to the team, was how selective the graphene was in targeting silver.
The quality of both the recovered and synthesized materials was then demonstrated in a silver-enhanced SPE dopamine sensor device, which was said to outperform two reference dopamine sensors made without the silver graphene composite
The team began the research by synthesizing graphene “using downstream microwave plasma” in atmospheric conditions. “The primary components of the system include a 2.45 GHz microwave generator, a matching network, a cooling system, and a reaction chamber,” their work stated.
A Raman spectrum analysis of the graphene showed “a characteristic 2D peak” at microwave powers between 200 W and 1000 W. “The images of transmission electron microscopy revealed interstitial spacing of 0.34, which matched the value of X-ray diffraction calculated through Bragg's law,” said the team.
The team then recovered silver from organic PV devices via leaching in a nitric acid solution. The PV coating contained indium tin oxide (ITO), zinc oxide (ZnO), molybdenum oxide (MoO3), and silver (Ag).
After the leaching was complete, the solution was cooled and used as a stock solution to create a graphene-coated SPE. “Following 10 min of electrodeposition, the Ag concentration slightly decreased to 1.69 ppm. This decrease suggests that some Ag ions were being reduced and deposited onto the electrode surface during the electrochemical process. After 20 min of electrodeposition, the Ag ions concentration further decreased to 1.62 ppm, indicating a continuous reduction in the Ag ion concentration,” said the team.
“These results suggest that a longer duration of electrodeposition may lead to further reduction in the silver concentration”. The Ag deposition was confirmed with cyclic voltammetric detection.
The team stated that the study “highlights the remarkable effectiveness” of graphene in recovering valuable metals like silver from e-waste.
To illustrate the quality of the composite material in a real-world application, the team made a graphene-silver electrode (SPE/graphene-Ag) detector and compared it to a bare SPE detector and a graphene/SPE detector. Test results showed that the SPE/graphene-Ag electrode displayed “significant improvement in peak current” compared to the other two specimens.
Other applications of graphene-silver composites were suggested by the researchers, such as corrosion-resistant coatings, conductive inks to use flexible devices in the electronics industry, antimicrobial coatings for use in biomedical industries, as well as sensors to detect gases, biomolecules, and pollutants.
The next steps for the team are to optimize the green synthesis process to improve its scalability and economic viability, aiming for a process that can be integrated into existing PV recycling and e-waste infrastructure.